Reaction Documentation for
The Reactions of RO2 with NO3
On the basis of reported information, these reactions are assumed to proceed via a single channel, as follows:
RO2 + NO3 → RO + NO2 (1)
Based on the data of Biggs et al. (1994), Daele et al. (1995) and Helleis et al. (1996), a value of k1 = 1.1 × 10-12 cm3 molecule-1 s-1 is assigned to the reaction involving CH3O2. A value of 2.5 × 10-12 cm3 molecule-1 s-1 is used for C2H5O2, (based on the reported coefficients of Biggs et al., 1995 and Ray et al., 1996), and this value is also used for the reactions of non-acyl peroxy radicals with NO3 in general. The rate coefficients for reactions of acyl peroxy radicals are assigned a value of k1 = 4.1 × 10-12 cm3 molecule-1 s-1, based on the reported value for CH3C(O)O2 (Canosa-Mas et al., 1996).
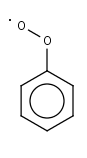 C6H5O2
C6H5O2
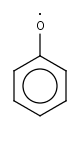 C6H5O
C6H5O