Reaction Documentation for
The Reactions of RO2 with HO2
Available rate coefficients for the reactions of RO2 with HO2 have been reviewed by Lightfoot et al. (1992), Wallington et al. (1992; 1997), Atkinson et al. (1999) and Tyndall et al. (2001), with recommendations made in a number of cases. Where applicable, the kinetic data applied to these reactions in the MCM are consistent with these recommendations. Where no experimental data are available, generic rate coefficients are defined by analogy. For acyl peroxy radicals and α-chlorinated peroxy radicals, the rate coefficients are assumed equivalent to those recommended by Atkinson et al. (1999) for CH3C(O)O2 and CH2ClO2, respectively. In all other cases, the rate coefficients are given by the expression:
where n is the carbon number. This expression was defined on the basis of room temperature data for the available series of reactions of various alkyl and β-hydroxy RO2 radicals with HO2 (Atkinson 1999, Rowley et al., 1992, Jenkin and Hayman (1995), Boyd et al., 1996 and Lesclaux et al., 1998). The temperature dependence is based on reported values for > C2 alkyl and β -hydroxy RO2 radicals (Rowley et al., 1992; Boyd et al., 1996).
The following channels are considered for the reactions of RO2 with HO2:
| RO2 + HO2 | → | ROOH + O2 | (1a) |
| → | ROH + O3 | (1b) | |
| → | R-HO + H2O + O2 | (1c) |
For the majority of peroxy radicals, the reaction is assumed to proceed exclusively by channel (1a), which is consistent with published data (Lightfoot et al., 1992; Wallington et al., 1992). For acyl peroxy radicals (RC(O)O2), branching ratios k1a/k1 = 0.71 and k1b/k1 = 0.29 are assumed, on the basis of the available data for CH3C(O)O2 and C2H5C(O)O2 (Lightfoot et al., 1992). In the case of peroxy radicals of general formula ROCH2O2, branching ratios k1a/k1 = 0.6 and k1c/k1 = 0.4 are assigned on the basis of data reported for HOCH2O2 (Burrows et al., 1989) and CH3OCH2O2 (Wallington et al., 1993).
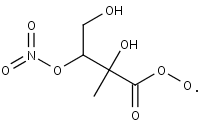 INDHCO3
INDHCO3
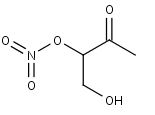 MVKNO3
MVKNO3