Reaction Documentation for
The permutation reactions of RO2
The "permutation" reactions of a given RO2 radical are its self reaction (1), and its cross reactions (2) with other peroxy radicals, R′O2, for which a number of product channels:
| RO2 + RO2 | → | RO + RO + O2 | (1a) |
| → | R-HO + ROH + O2 | (1b) |
| RO2 + R′O2 | → | RO + R′O + O2 | (2a) |
| → | R-HO + R′OH + O2 | (2b) | |
| → | R′-HO + ROH + O2 | (2c) |
Available rate coefficients for the permutation reactions of RO2 have been reviewed by Lightfoot et al. (1992), Wallington et al. (1992), Lesclaux (1997), Atkinson et al. (1999) and Tyndall et al. (2001), with recommendations made in a number of cases. In view of the large number of RO2 radicals generated in a detailed chemical mechanism, however, it is unrealistic to represent these reactions explicitly, and the use of a simplified parameterisation is essential (Madronich and Calvert, 1990). A very simplified approach is adopted in the MCM, in which each peroxy radical is assumed to react with all other peroxy radicals (i.e. the peroxy radical "pool") at a single, collective rate (Jenkin et al. 1997). This is achieved by defining a parameter ΣRO2 which is the sum of the concentrations of all peroxy radicals, excluding HO2. The collective rate of all the permutation reactions of a particular peroxy radical is then represented by a pseudo-unimolecular reaction, which has an assigned rate coefficient equal to k3.ΣRO2:
| RO2 | → | RO | (3a) |
| → | R-HO | (3b) | |
| → | ROH | (3c) |
Each reaction has up to three product channels, the branching ratios of which depend on the structure of the radical, as shown in Table 1.
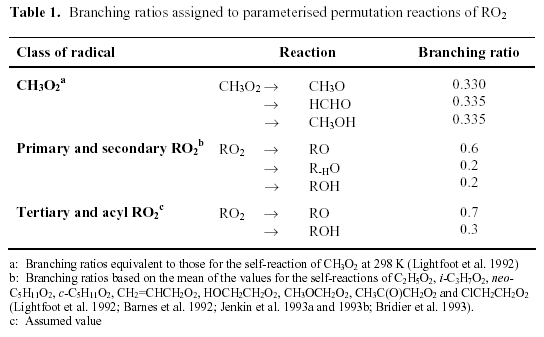
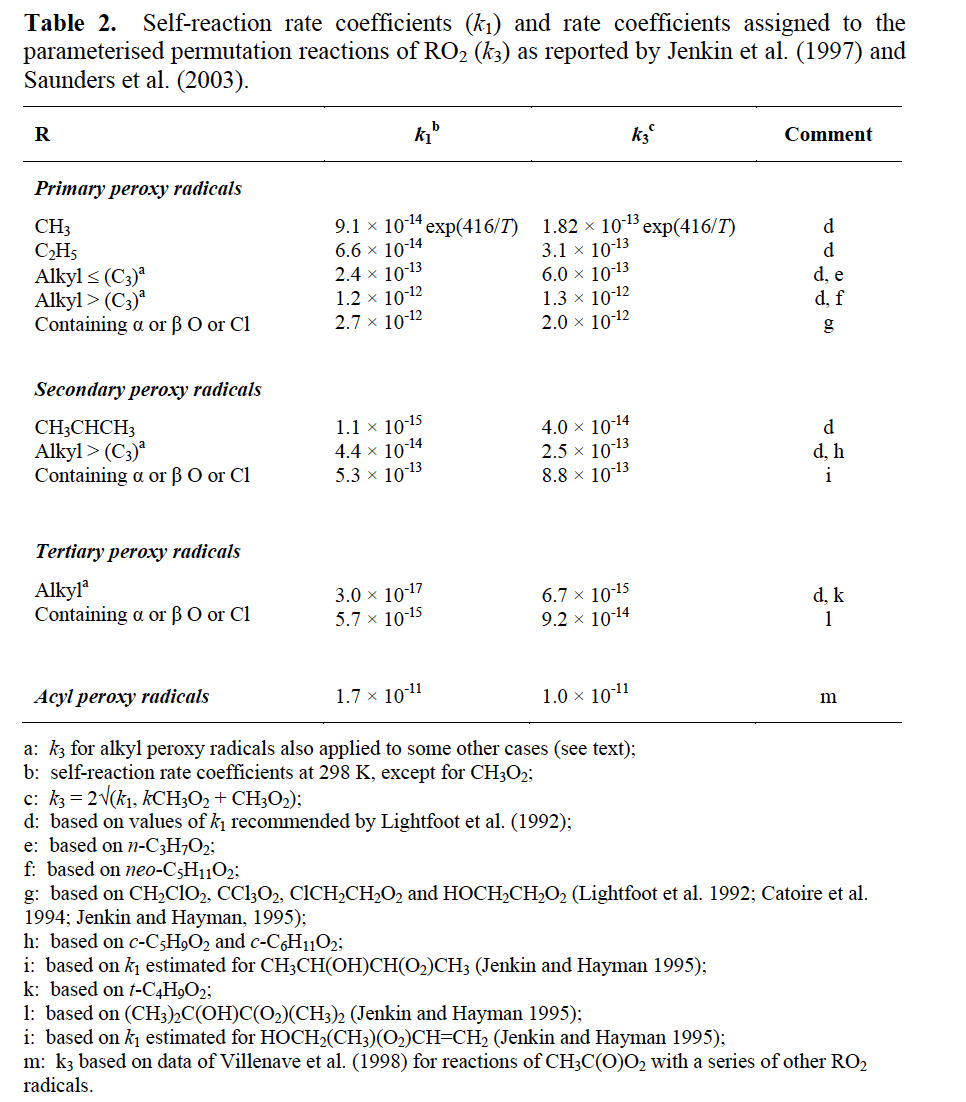
The assigned values of k3 for various classes of peroxy radical (as given in Table 2) also depend on the structure of the organic group, and are based on trends of reactivity of peroxy radical self reactions. The parameters are twice the geometric mean of an estimated self-reaction rate co-efficient, k1, for that class of peroxy radical and the self-reaction rate coefficient for CH3O2 (i.e. it is assumed that CH3O2 is the major reaction partner). Accordingly, the representation of the reaction for CH3O2 itself reduces to its self-reaction with recommended rate coefficient and branching ratios, when it is the dominant radical. The rate coefficients for alkyl peroxy radicals are also taken to apply to remotely substituted (i.e. not α or β) oxygen- and chlorine-containing radicals, radicals containing unsaturated linkages, and β -nitro-oxy peroxy radicals.
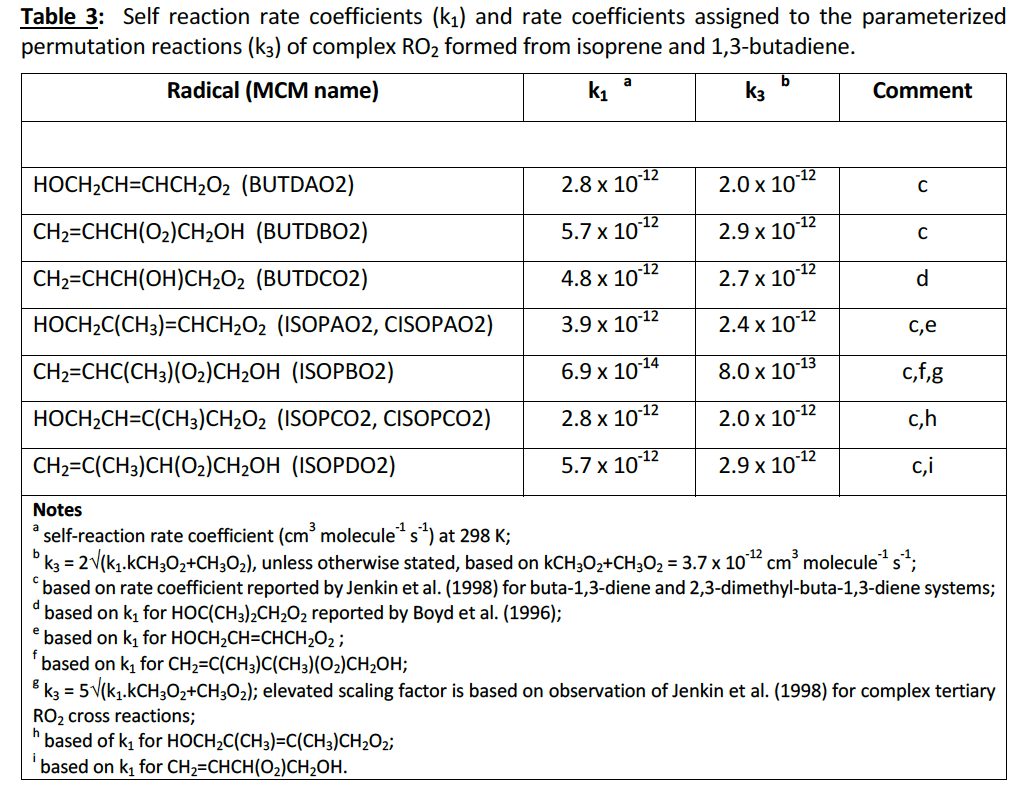
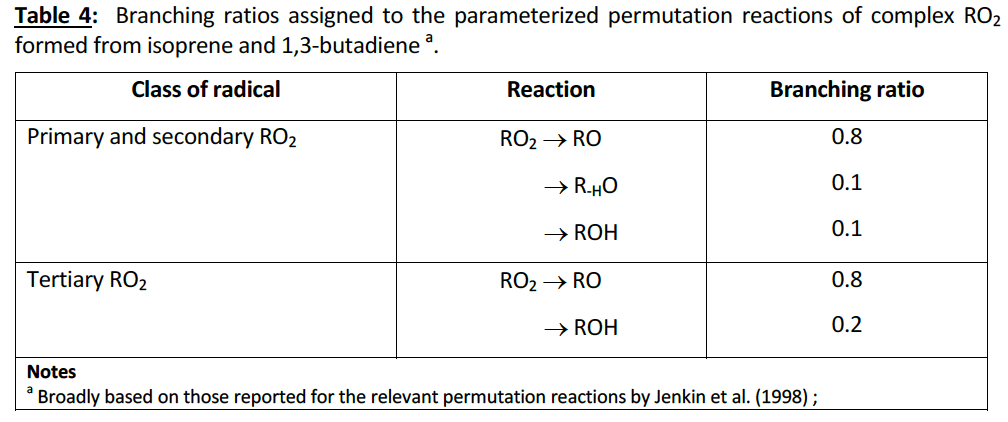
For peroxy radicals generated during the oxidation of isoprene and 1,3-butadiene (which contain combinations of allyl and β-hydroxy groups) have been increased to the values given in Table 3 and Table 4. These are based on data summarised by Jenkin et al. (1998) for RO2 radicals generated in these systems.
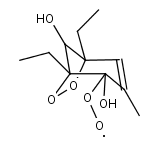 DEMPHOLO2
DEMPHOLO2
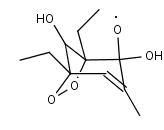 DEMPHOLO
DEMPHOLO