Reaction Documentation for
The Reactions of RO2 with NO2
These reactions are only treated in the MCM for acyl peroxy radicals, for which the product peroxy nitrates (ROONO2) are comparatively stable, and for the most abundant peroxy radical, CH3O2:
RO2 + NO2 (+M) ↔ ROONO2 (+M) (1, -1)
Available rate coefficients for the forward and reverse reactions have been reviewed by Lightfoot et al. (1992), Wallington et al. (1992; 1997), Atkinson et al. (1999) and Tyndall et al. (2001). In the absence of rate data for the acyl peroxy radical forward reaction (1), and the reverse peroxy nitrate decomposition reaction (-1) the rates are assumed equivalent to that for CH3C(O)O2 and CH3C(O)OONO2 respectively.
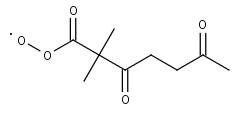 C827CO3
C827CO3
 C827PAN
C827PAN