Reaction Documentation for
NO3 initiation reactions
NO3 initiated degradation chemistry is included for those VOC for which both the following relations apply:
These relations were formulated on the basis that typical boundary layer concentrations of OH and NO3 are 106 and 109 molecule cm-3, respectively. Thus, the first criterion applies when the removal rate by reaction with NO3 exceeds 1% of the removal rate by reaction with OH, and the second when the lifetime of the VOC with respect to reaction with NO3 is less than 107 s (ie. ca. 100 days). NO3 initiated degradation is therefore believed to be important for alkenes, dienes, aldehydes and ethers.
Rates of initial reactions
Rate coefficients for the reactions of NO3 with organic compounds have been reviewed by Atkinson (1991; 1994; 1997a), Wayne et al. (1991), Atkinson et al. (1999) and Calvert et al. (2000). The reviews of Atkinson and Calvert also make recommendations in many cases. These recommendations are used unless superseded by more recent evaluations. If recent laboratory determinations are available, which are likely to influence, or form the basis of future recommendations, these are also taken into account. The reactions of NO3 with aldehydes for which no experimental data exist, are assigned an appropriate generic rate coefficient based on the data presented in Table 1, which makes use of a substantial new body of kinetic data on this class of reaction (Papagni et al., 2000; Ullerstam et al., 2000; DAnna et al., 2001). The generic coefficients allow for the observed significant increase in the rate coefficient with increasing carbon number and branching for the series of aldehydes considered in these studies.
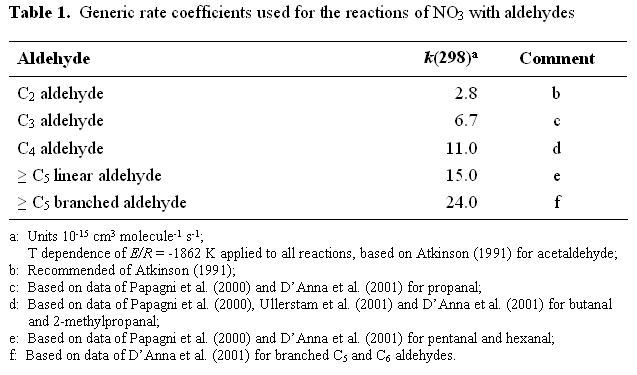
The kinetics of the reactionsof NO3 with dimethylether, diethyl ether, di-n-propyl ether and methyl t-butyl ether have been studied by Langer and Ljungström (1994), and the reported rate coefficients are used accordingly. Partial rate coefficients for methyl, ethyl, n-propyl and t-butylgroups of 0.13, 1.40, 3.25 and 0.51 ( in units of 10-15 cm3 molecule s-1) may be inferred from these data, and were used for these groups and related hydroxy substituted groups in the ethers and glycol ethers considered in the present work. In the absence of experimental data, the partial rate coefficient for the n-butyl group is assumed equivalent to that for the n-propyl group.
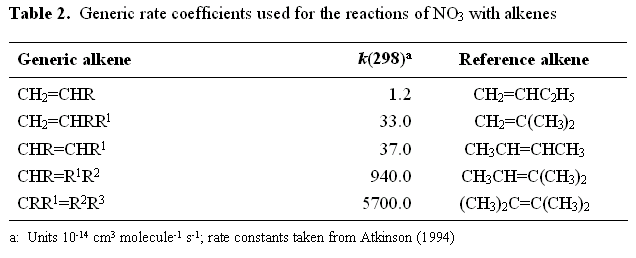
Rate coefficients for the reactions of NO3 with monoalkenes increase dramatically with alkyl substitution (Atkinson, 1991, 1994; Wayne et al. 1991). Table 2 lists a series of generic rate coefficients, which were estimated by analogy. These are used in the absence of available experimental data.
Initial radical products
The reactions of NO3 with aldehydes are assumed to proceed via abstraction of the aldehydic H-atom, leading to the production of acyl radicals. The reactions with ethers and glycol ethers are also assumed to result in H-atom abstraction.
The attack of NO3 on alkenes, dienes and monoterpenes is assumed to proceed by an addition mechanism, leading to the formation of β-nitro-oxy-substituted alkyl radicals. In the present work, the addition of NO3 to alkenes of generic formulae CH2=CHR, CH2=CRR1 and CHR=CR1R2 is assumed to occur on the less alkyl substituted carbon 65, 80 and 65% of the time, respectively. Thus, in the case of CH2=CHR:
| NO3 + CH2=CHR | → | CH2(ONO2)CHR (65%) | (3a) |
| → | CH2CH(ONO2)R (35%) | (3b) |
For unsymmetric alkenes of generic formula CHR=CHR1 or CRR1=CR2R3, NO3 is assumed to add equally at both sites. On the basis of the data of Skov et al. (1992), the addition of NO3, to 1,3-butadiene is assumed to occur only at the terminal carbon atoms, and in the case of isoprene, entirely at the terminal carbon adjacent to the methyl substitution.
 DMS
DMS
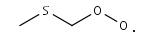 CH3SCH2O2
CH3SCH2O2